New regulations for medical devices: The impact of the Medical Device Regulation (MDR)
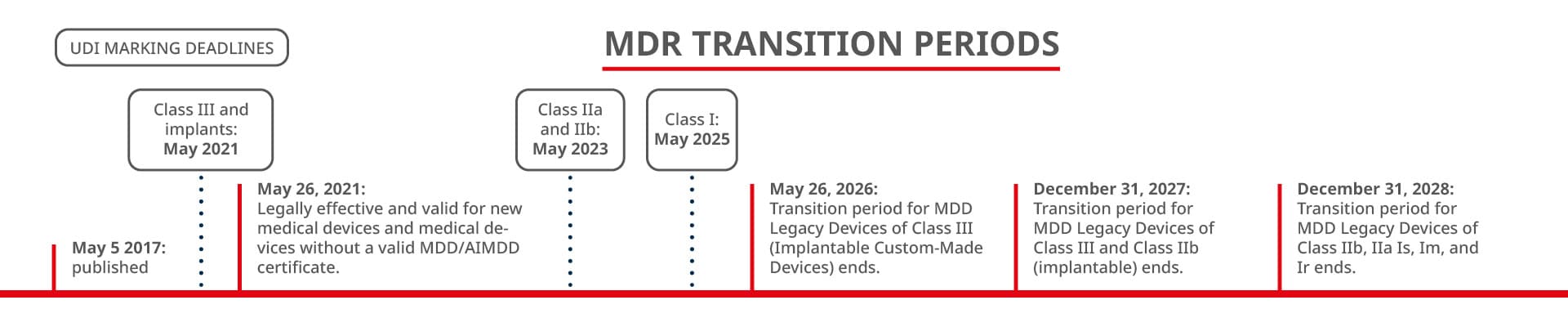
On May 26, 2021, the Medical Device Regulation (MDR) of the European Union became binding, bringing changes for all manufacturers of medical products. But what exactly do these new regulations entail?